
Welcome!
We provide some ready-made examples to demonstrate ENZO capabilities. After you open an example project, take a look at the reaction scheme and the parameters. You do not have to change anything.
When you are ready press "Start". Follow the accordance between theoretical and experimental curves. Current parameters are shown for each iteration.
Of course, you can try changing the parameters and see how the evaluation progress changes.
Example1: Active site concentration and enzyme activity correlation
We titrated the active site of Torpedo californica acetylcholinesterase (TcAChE) using m-(N,N,N-trimethylammonio) trifluoroacetophenone (NAF), a transition state analogue with affinities reported to be in the atomolar range. The reaction between the TcAChE and NAF is theoretically reversible, but due to the high NAF affinity for the active site, it can be regarded as essentially irreversible, and written as the following reaction scheme:
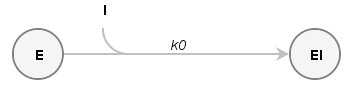
Example2: Autoactivation of Procathepsin B
Reaction scheme for autoactivation with product inhibition:
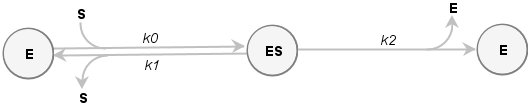
Example3: Cholinesterase reaction with butyrylthiocholine
Due to specific structural properties, such as a buried active site, a peripheral substrate binding site and a backdoor channel, cholinesterases are highly efficient and specific, but in general, they do not obey hyperbolic Michaelis-Menten kinetics in their reactions with substrates. The active site in cholinesterases is relatively large and a second substrate molecule can bind to the peripheral site at different times before the turnover of the first substrate is completed at the catalytic site. All this information can be easily drawn as a comprehensive reaction scheme:
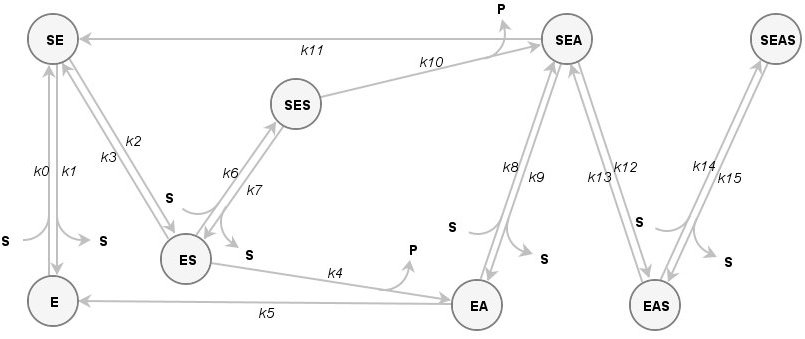
of Kinetic Models of Enzyme Catalyzed Reactions. PLoS ONE 6(7): e22265. doi: 10.1371/journal.pone.0022265 (Open Access, BibTeX)

